| CLT-ACID | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 88-53-9 |
|
| EINECS NO. | 201-839-7 | |
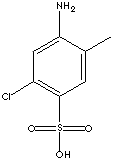
| FORMULA | C7H8ClNO3S | |
| MOL WT. | 221.66 | |
| H.S. CODE |
| |
| TOXICITY |
| |
| SYNONYMS | Cassella Acid; | |
| 2-Amino-5-chloro-4-methylbenzenesulfonic acid; | ||
| 2-Amino-5-chloro-4-methylbenzenesulfonic acid; Lake Red C Amine; 6-Chloro-m-toluidine-4-sulfonic acid; C-Acid; | ||
| SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | White to light redish powder | |
| MELTING POINT |
| |
| BOILING POINT | ||
| SPECIFIC GRAVITY |
| |
| SOLUBILITY IN WATER | ||
| AUTOIGNITION | ||
| NFPA RATING | ||
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions | |
|
APPLICATIONS |
||
|
Toluidine is the
trivial name of methylaniline or aminotoluene. There are three structural
isomers, ortho-TOLUIDINE, meta-TOLUIDINE, and
para-TOLUIDINE. The names of the three compounds indicate amino
group positions relative to the methyl group on the benzene ring. They can be
obtained from coal tar as by-products in the fractional distillation or can be
prepared by reducing nitrotoluene which are made by nitration of toluene.
Toluidine is a compound homologous with aniline (the simplest aromatic amine)
and similar properties aromatic amine. Toluidine is not soluble in water but in
acidica queous solution. o-Toluidine and m-Toluidine are clear to pale yellow,
oily liquid. But p-Toluidine solidifies to an off-white crystalline solid at
about 43 C as it has symmetrical structure . They are sensitive to air and light
and tend to darken on storage. They are toxic if inhaled, ingested, or absorbed
through the skin. Typical end-use markets for the toluidines and their
derivatives are main materials for the synthesis of imaging products like
pigment, dyestuffs,and photographic chemicals. All of these compounds are also
used in the production of antioxidants, agricultural, pharmaceutical and rubber
chemicals.
Intermediate for dyes and organic pigments especially for red color. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
White to light reddish powder | |
| PURITY |
98.0% min | |
| MOISTURE | 1.0% max | |
|
ASH |
1.0% max | |
|
INSOLUBLES IN ALKALI |
0.1% max | |
| TRANSPORTATION | ||
| PACKING | 25kgs in Bag | |
| HAZARD CLASS | ||
| UN NO. | ||
| REMARKS | ||
|
|
||